How To Determine The Hybridization
You will be able to download the video notes and admission practice questions when you start your site membership.

Organic Chemical science Tutor Site Membership
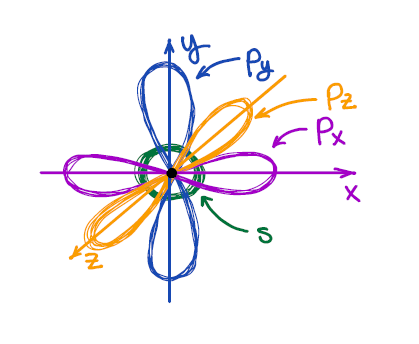
We all know from general chemistry that the south-orbital is spherical, and p-orbitals are dumbbell-looking orbitals oriented along the ten, y, and z axes of the Cartesian organisation. Nosotros also know that VSEPR describes the 3D shape of the 2nd period elements reasonably well.
And here we take a problem: the atomic orbitals are at 90° to each other, while the VSEPR theory predicts the 3D structure of, say, marsh gas (CH4) to be tetrahedral with bond angles effectually 109.5°. So, how can we have 109.5° bond angles made by the orbitals which are at 90° to each other? 🤯🤷♂️🤔
In 1931 the twice Nobel Laureate Linus Pauling proposed the model of "mixing" the orbitals or "hybridizing" them to account for the observed bonding pattern. Pauling proposed sort of a combination of the orbitals giving you an orbital that has partial characters. Not a complete southward- or a p-orbital, merely rather something with a partial south- and fractional p-character.
Let me emphasize ane more than time that hybridization is a mathematical model . In that location'due south no bodily "process" that happens to orbitals that causes the hybridization. The atomic orbitals don't actually alter earlier going into the bonding with other atoms. Hybridization is a mathematical model that describes how the diminutive orbitals would've looked similar based on the appreciable molecular orbitals.
Formation of the Hybridized Orbitals
Ok, at present when we know that hybridization is a model and not an actual process, let's expect at how this "process" happens. 🤣 Each bail takes 2 electrons to complete. If nosotros expect at the carbon atom atomic orbitals, we'll see the two electrons on the 2s and two electrons on the 2p shells. This would only let carbon to make 2 bonds since information technology only has two unpaired electrons. To make four bonds, carbon would take to "decouple" its s-electrons onto the p shell.
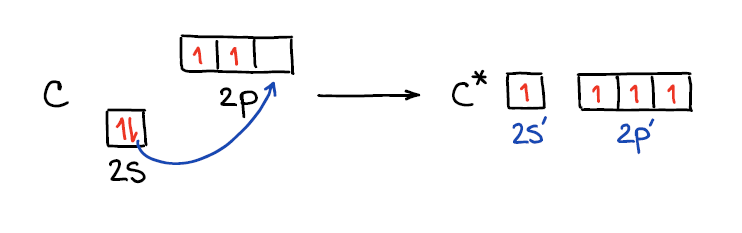
Rearranging the electrons in an cantlet in this way likewise makes the orbitals closer in energy making them virtually degenerate. This allows for easier "mixing" or hybridization every bit we know it. Thus, when nosotros mix those orbitals together we end upwards with a gear up of "hybrids" and any leftovers that were not hybridized.
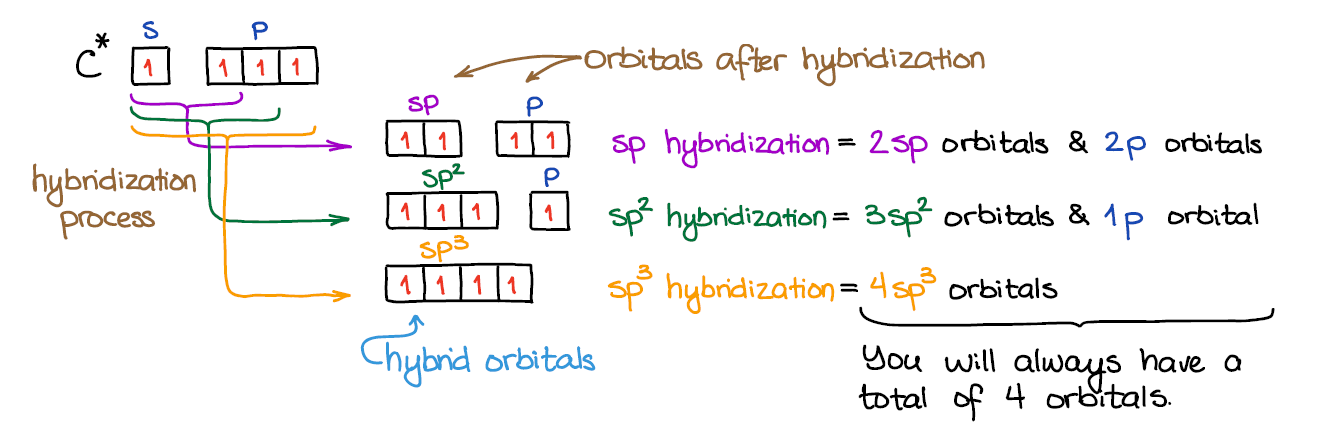
An important thing to call up: # of AO's = # of MO. So, when nosotros mix the diminutive orbitals to make the hybrids, we will end upward with the exactly the aforementioned number of the the orbitals when nosotros're washed. Thus, by mixing four orbitals (one s and three p), we'll e'er get 4 molecular orbitals (hybrids or not).
sp3 Hybridization
Mixing an s-orbital and three p-orbitals gives four sp3 orbitals.
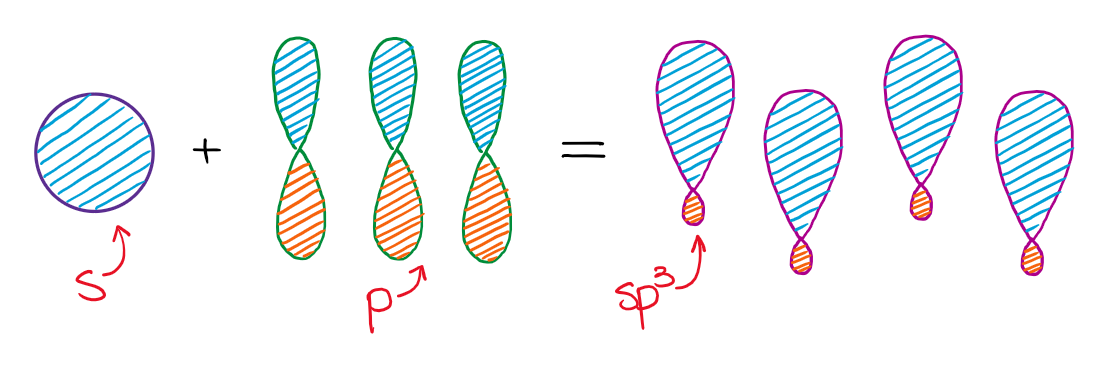
The resulting four hybrid sp3 orbitals are all degenerate in energy, significant they are all the same. As well, co-ordinate to VSEPR theory, those orbitals need to be as symmetric around each other as possible. This gives a tetrahedral structure with bond angles effectually 109.5°.
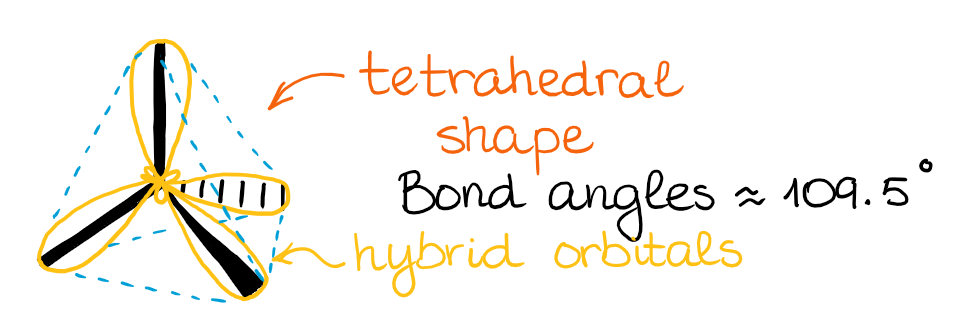
When those hybrid orbitals make bonds, we get molecular orbitals oriented in the same direction. So, as I've mentioned earlier, while the hybridization and the hybrid orbitals might be the mathematical model, it does help us predict and illustrate the actual molecular orbitals in the molecule. BTW, the molecular orbital theory (MOT) is a mathematical model too. 🤣 Nonetheless, we can perform calculations using the MOT to predict the electron densities around the molecule congruent with the real physical observations.
sp2 Hybridization
When we mix one s-orbital and two p-orbitals, nosotros get three sp2 hybridized orbitals. You'll also take one leftover p-orbital that didn't participate in the hybridization.
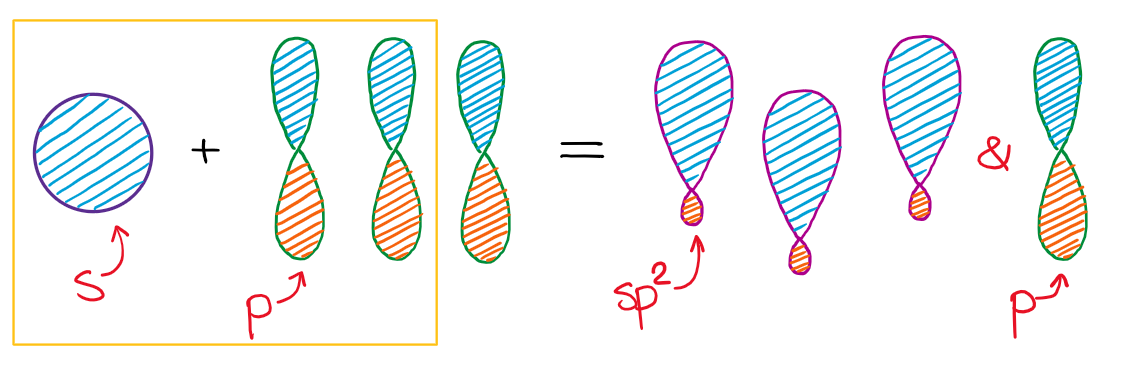
This hybridization gives you the trigonal planar geometry around the central atom with the p-orbital sticking in the upward and down vertical management.
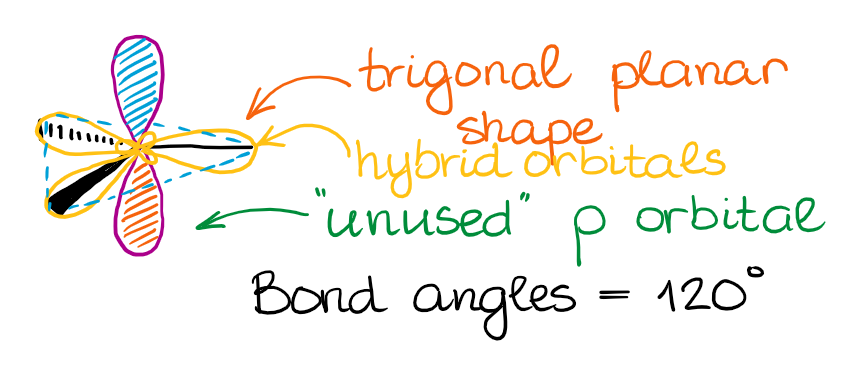
The "unused" p-orbital can brand a π-bond or to participate in a complex resonance conjugation.
sp Hybridization
In the instance of the sp hybridization, simply one southward- and 1 p-orbital are mixed together to make hybrids. This leaves two unused p-orbitals.
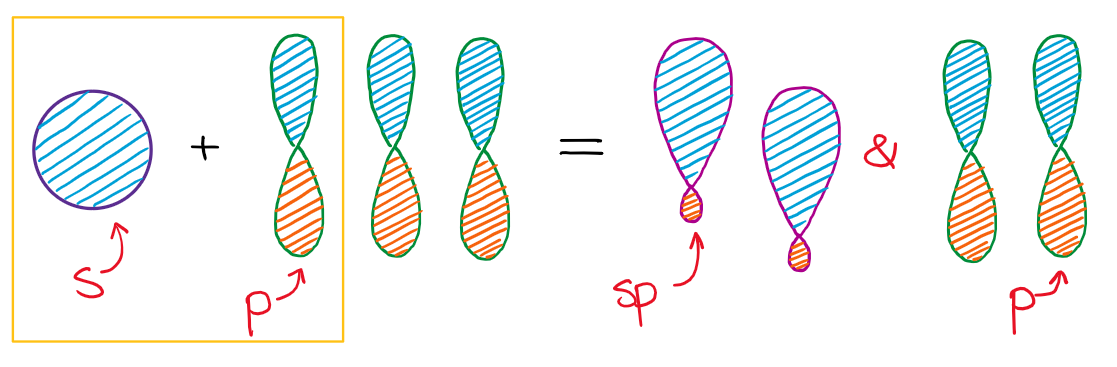
The unused sp orbitals strength the construction to take a linear 3D geometry.
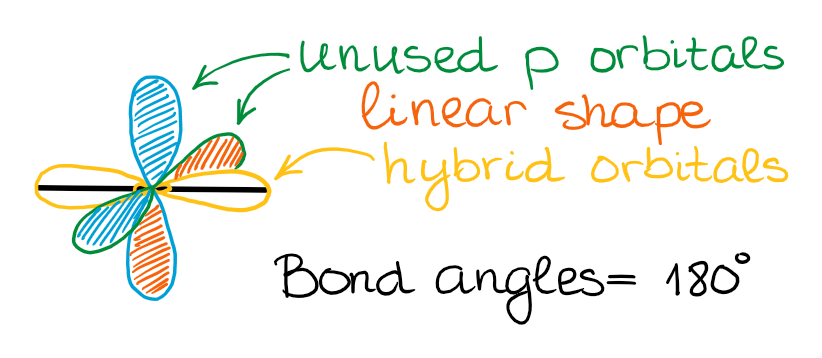
The unused p-orbitals can make ii double bonds, a triple bail, or potentially participate in resonance with other orbitals.
How Practise Nosotros Determine Hybridization?
The easiest way to determine hybridization is to with the VSEPR theory and decide the number of electron groups around your fundamental atom. To put it plain, I tin summarize the hybridizations in the post-obit film:
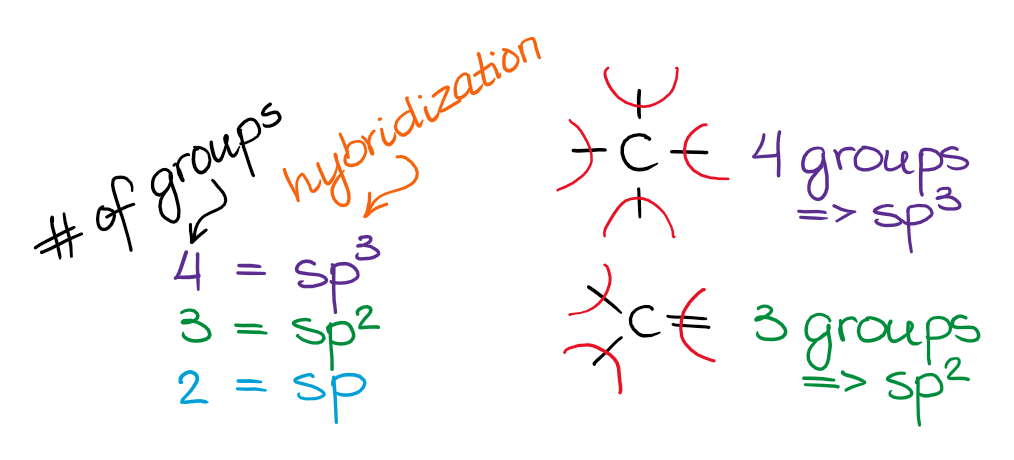
And so, the 3 groups effectually the key cantlet gives you the sp3 hybridization, the three groups gives you sp2 hybridization, and the two groups yield the sp-hybridized species. Also call up, nosotros practise count the the spare electron pairs every bit the electron groups too!
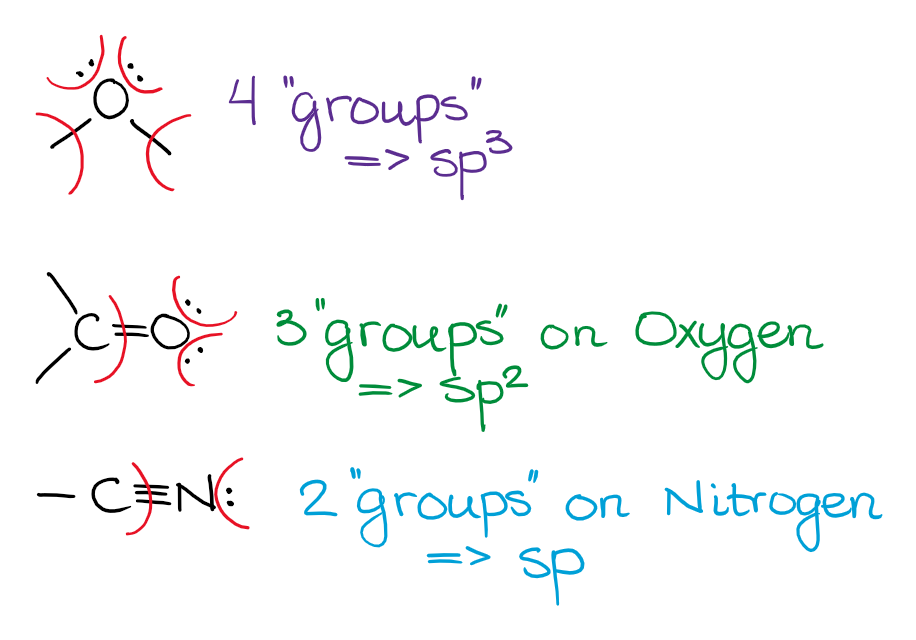
Unless the electron pair is next to a double or a triple bond (or an empty p-orbital), the electron pair will be on the hybrid orbital and not the p-orbital. While that is not 100% truthful in reality, that'due south the way we treat it inside the scope of a typical organic chemistry form, so we'll stick with it too.
Hybridization of Atoms with Electron Pairs next to Double or Triple Bonds
I've mentioned above, that a double or a triple bond next to an electron pair matters. When you accept an electron pair side by side to a p-orbital or a π-bail, there's a resonance between those.
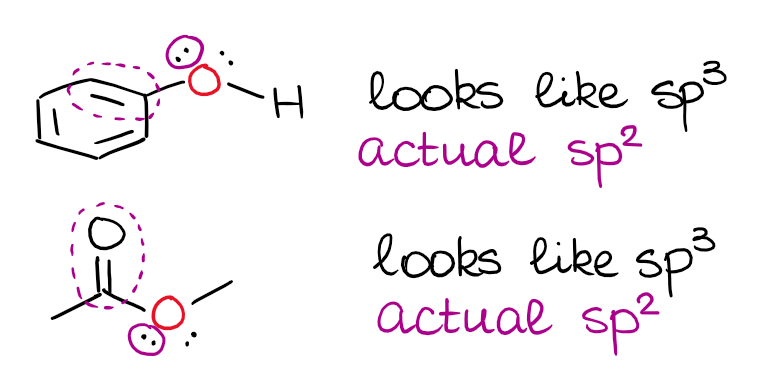
This is a very typical "trick question" on the exam, and then you wanna keep this in mind. It'southward also important to retrieve that the electron pair has to be physically able to align with the p-orbital or a π-bond for this to happen. Then, the isolated electron pairs will nonetheless exist sitting on the hybrid orbitals even when they are next to double bonds.

Spotting the isolated electron pairs can be a little tricky, then you lot may wanna exercise some practise to principal this skill. At that place is a quick dominion of pollex you can use. If you have an electron pair on the atom that already has a double bail, chances are, it's going to be isolated. It'south not a 100% foolproof flim-flam, just information technology works for cyclic structures.
Are you ready to tackle some practice questions?
How To Determine The Hybridization,
Source: https://www.organicchemistrytutor.com/topic/hybridization/
Posted by: hertzogdair1985.blogspot.com


0 Response to "How To Determine The Hybridization"
Post a Comment